Everbridge Group is progressively building a multidimensional product portfolio in the peripheral vascular domain, including balloons, guidewires, catheters, stents, accessories, devices, and consumables. This comprehensive offering covers all clinical scenarios in peripheral vascular interventions. Compared to traditional surgical interventions, peripheral vascular therapies offer distinct clinical advantages, such as being minimally invasive, providing significant therapeutic efficacy, and enabling rapid postoperative recovery. Additionally, these therapies result in lower risk and less procedural discomfort for patients. This innovative approach shows great potential for widespread clinical application in modern medical practice.
Ischemic
-
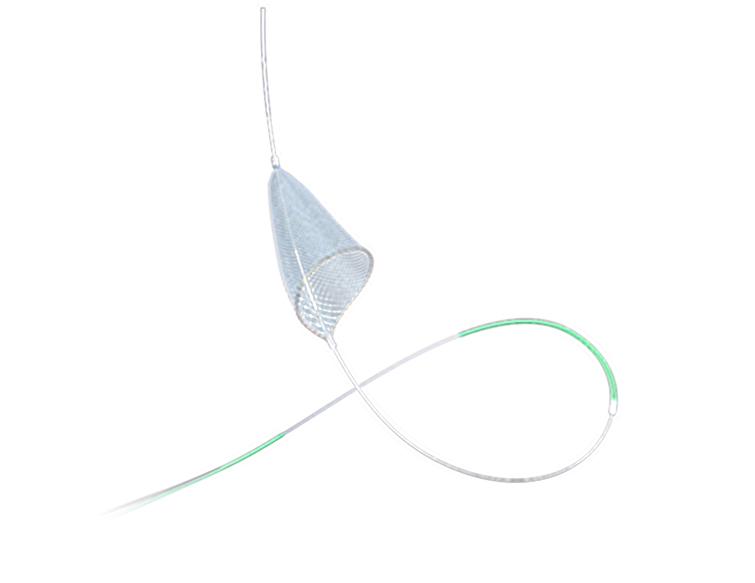
PathGuard Embolic Protection Device is intended for use in providing patients with protection from distal vascular embolization during carotid artery interventional procedure.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
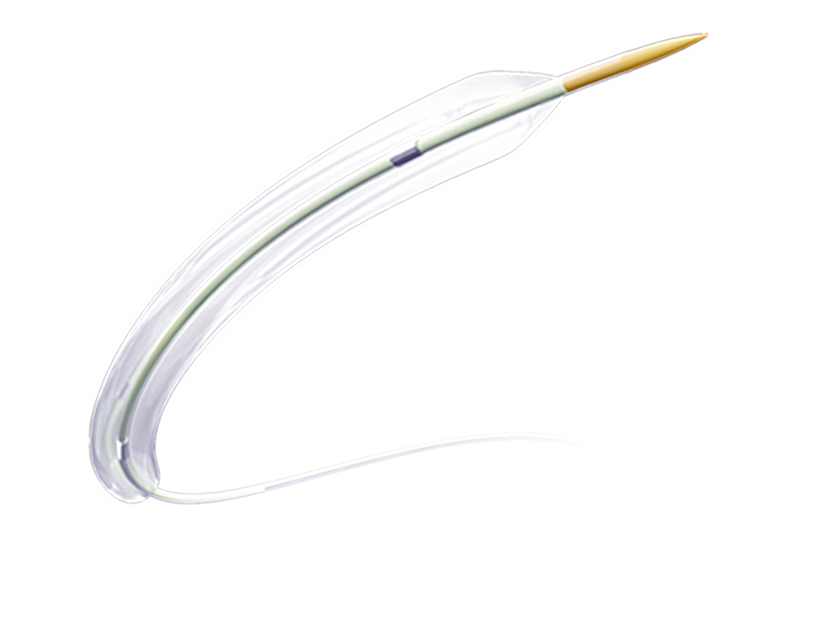
N-Balloon Intracranial Balloon Dilatation Catheter is Semi compliant balloon. It is suitable for interventional treatment of patients with non-acute symptoms of intracranial arterial atherosclerotic stenosis. Through the balloon dilatation, blood supply can be restored, and blood perfusion of intracranial arteries can be improved.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
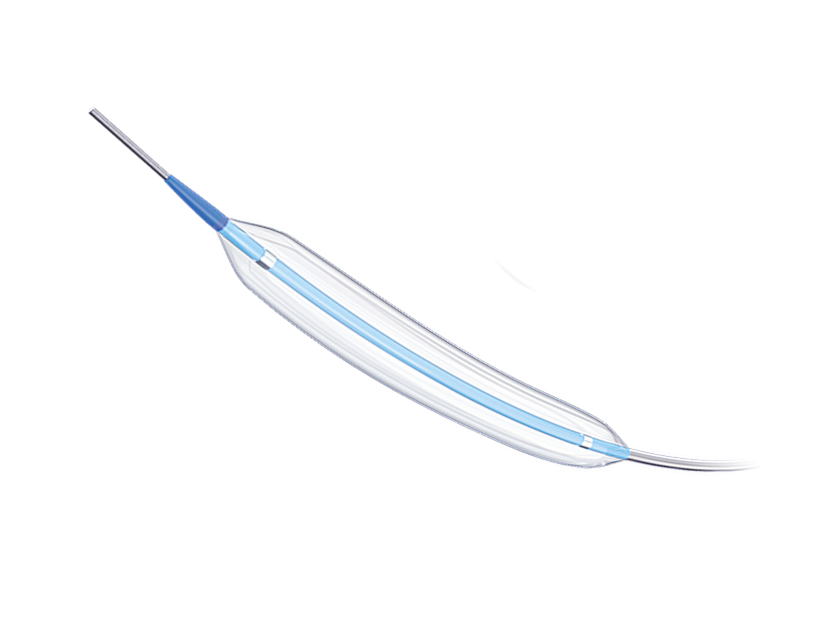
Balloonfish is Semi compliant balloon,it is suitable for interventional treatment of patients with non-acute symptoms of intracranial arterial atherosclerotic stenosis. Through the balloon dilatation, blood supply can be restored, and blood perfusion of intracranial arteries can be improved.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
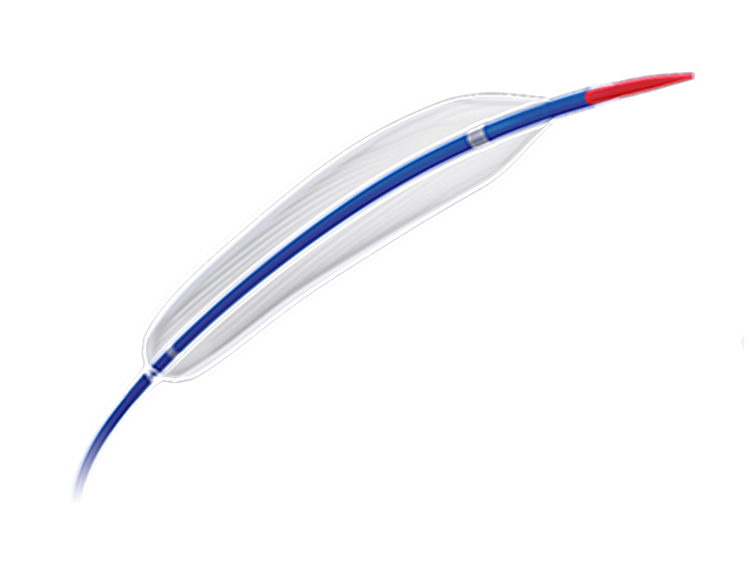
C-Balloon Carotid PTA Balloon Dilatation Catheter is Semi compliant balloon. Intended for use in percutaneous transluminal angioplasty in patients with carotid artery occlusive disease.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
This product is intended for recanalization in patients with acute ischemic stroke secondary to intracranial large vessel occlusion (internal carotid artery, middle cerebral artery-M1 and M2 segments, basilar artery, and vertebral artery).For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.



-
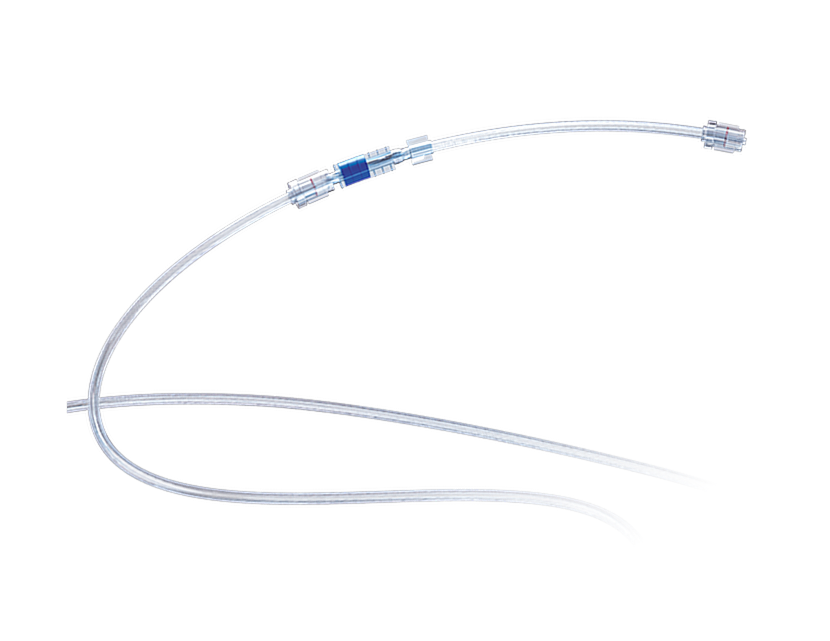
Combination with suction pump,and universal standard Luer connector for Better compatibility. Combination with suction pump.It is used for extracorporeal aspiration of waste fluids, blood and thrombus during surgery.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
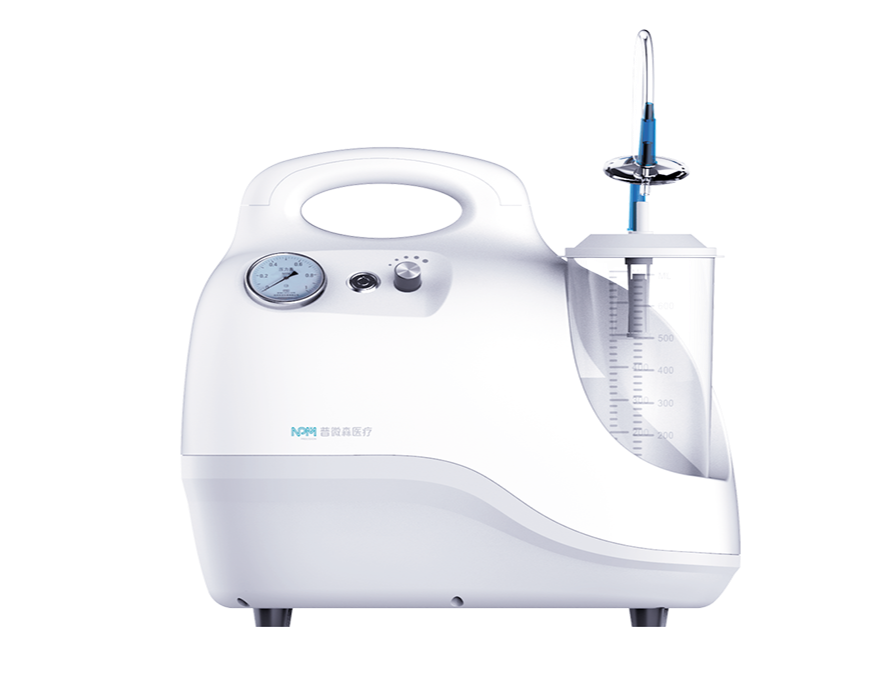
The combination of NewSped Negative Pressure Suction Device and lengthen Connecting Tube can cover the entire process of thrombus suction. It is used in coniunction with the suction extension tube for intraoperative suction of waste fluids, blood and thrombi.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-

 PathGuard Embolic Protection Device
PathGuard Embolic Protection Device
-

 N-Balloon Intracranial Balloon Dilatation Catheter
N-Balloon Intracranial Balloon Dilatation Catheter
-

 Balloonfish Intracranial Balloon Dilatation Catheter
Balloonfish Intracranial Balloon Dilatation Catheter
-

 C-Balloon Carotid PTA Balloon Dilatation Catheter
C-Balloon Carotid PTA Balloon Dilatation Catheter
-

 PGAspiration Intracranial Aspiration System
PGAspiration Intracranial Aspiration System
-

 lengthen Connecting Tube
lengthen Connecting Tube
-

 NewSped Negative Pressure Suction Device
NewSped Negative Pressure Suction Device
Access
-
The guide catheter is used to introduce interventional or diagnostic instruments into the peripheral and neurovascular system.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
The catheter is used to introduce the interventional or diagnostic instruments into the peripheral and neurovascular system.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.



-
This product is intended for general intravascular use, including neurovascular and peripheral vascular applications. The guidewire is compatible with diagnostic or therapeutic catheters to facilitate placement at the target lesion site.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
The support catheter is suitable for the introduction of diagnostic or therapeutic devices in the neurovascular and peripheral vascular systems.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.



-
It is suitable for asisting in the insertion of intravascular catheters and guiding them into the target vessel of the carotid artery.The balloon provides temporary vessel occlusion during angiography.In addition, the balloon guide catheter can be used as a channel for retrieval devices.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
Intended for use during interventional procedures to introduce guidewires, catheters, and other medical devices into the vasculature.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
PGCatheter Microcatheter is intended for use in neuroendovascular procedures and infusion of diagnostic agents (such as contrast agents) and therapeutic devices (such as stents, embolic coils).For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.



-
This product is intended for general intravascular use, including the neurovascular and peripheral vascular systems. The guidewire is designed to be used with diagnostic or therapeutic catheters to facilitate their placement at the selected lesion site.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-
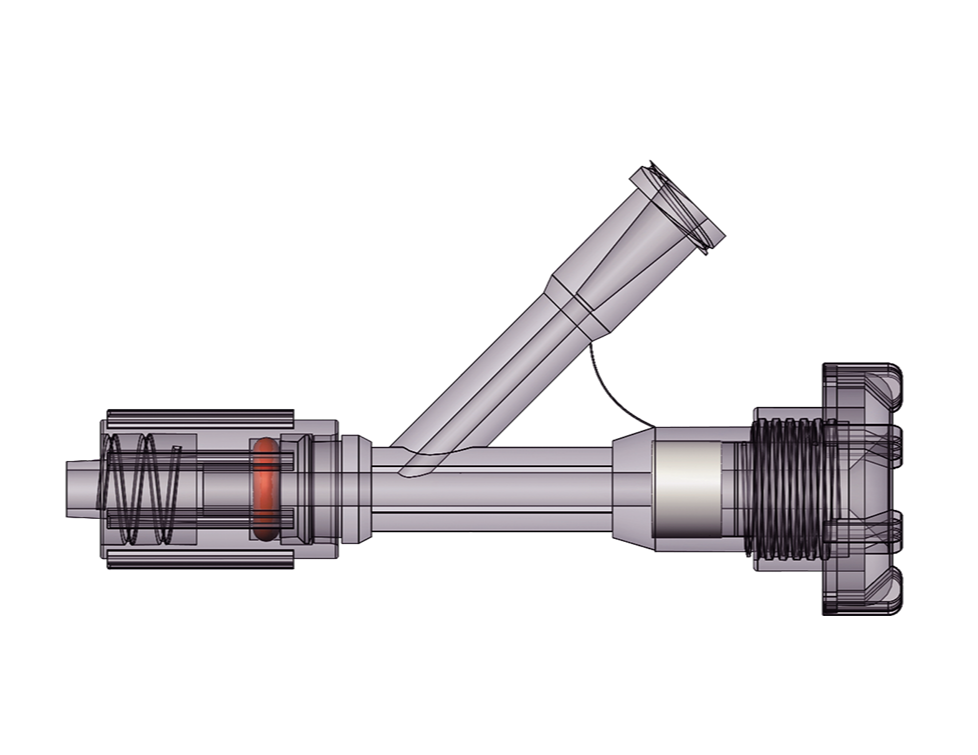
It is intended to be used in PTCA procedures to assist in establishing a working channel for guiding wires into the human outside the body.For detailed product information, the official product registration/licensing documentation for the respective market should be consulted.


-

 MidAccess Access Catheter
MidAccess Access Catheter
-

 MidAccess 088" Access Catheter
MidAccess 088" Access Catheter
-

 VasCruiser Distal Access Catheter
VasCruiser Distal Access Catheter
-

 Farender Support Catheter
Farender Support Catheter
-

 FlexBGC Balloon Guide Catheter
FlexBGC Balloon Guide Catheter
-

 PGGuider Long Sheath
PGGuider Long Sheath
-

 PGCatheter Microcatheter
PGCatheter Microcatheter
-

 SmartPioneer Guidewire
SmartPioneer Guidewire
-

 Fivalve Y-Connectors
Fivalve Y-Connectors